
New Drug Designations - October 2024
Shots:
- PharmaShots' designation report provides a concise overview of the latest drug designations by major regulatory authorities, including the FDA, NMPA, and EMA
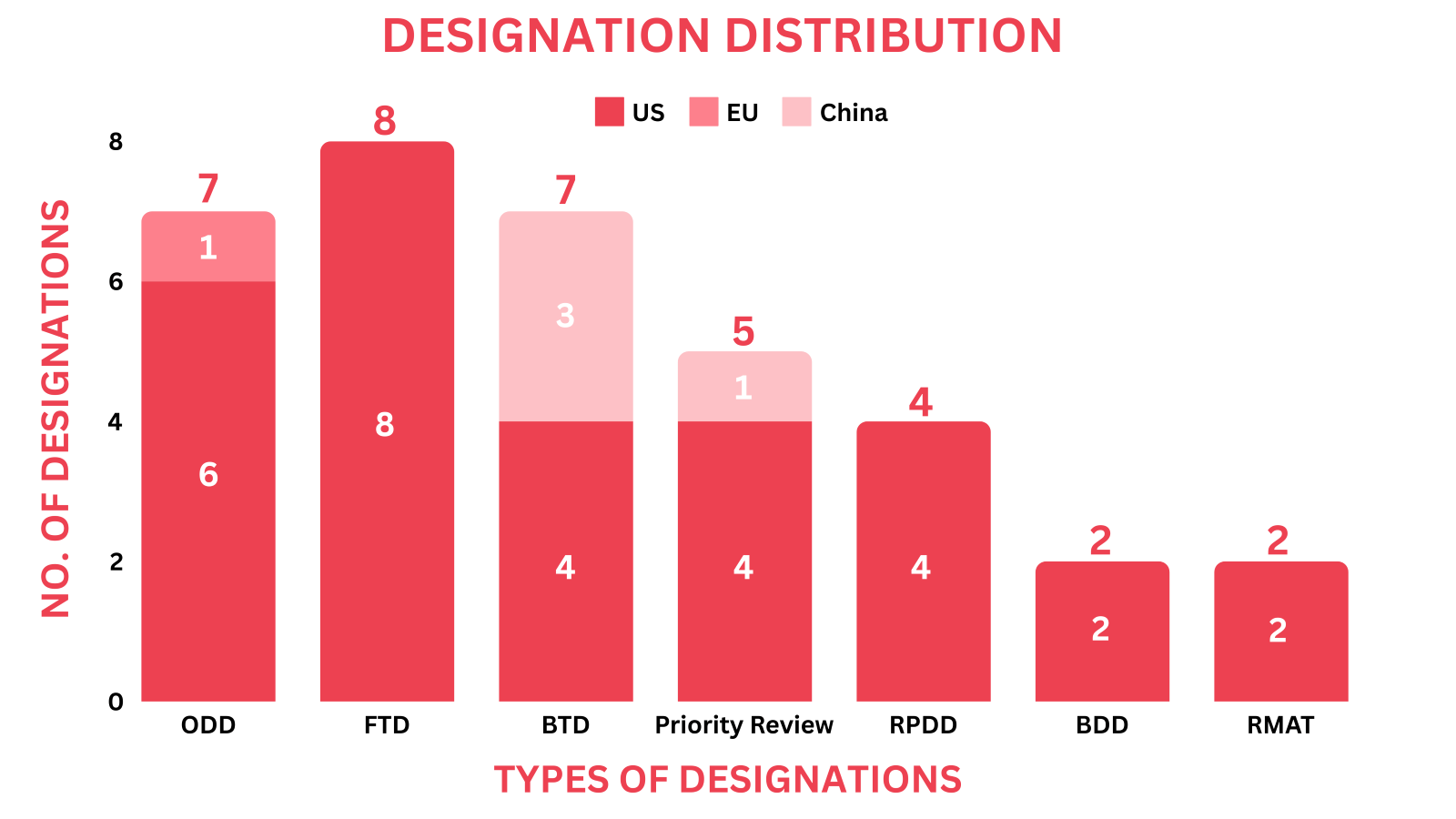
- The October 2024 report covers designations granted to 33 drugs and 2 devices, encompassing 14 small molecules, 4 biologics, 8 cell and gene therapies, and 2 medical devices.
- Significant trends this month show, Papillon Therapeutics' PPL-002 has received Orphan Drug Designation (ODD) and Rare Pediatric Disease Designation (RPDD) from the FDA for the treatment of Danon Disease, marking significant progress in addressing rare genetic disorders.

LQT-1213
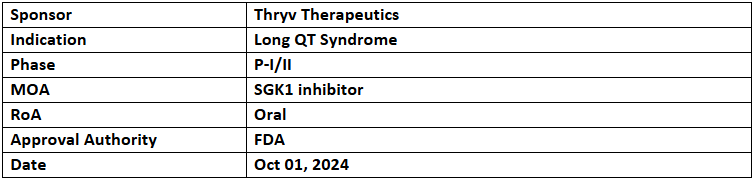
- The US FDA has granted ODD to LQT-1213 for the treatment of Long QT Syndrome, based on the ongoing Wave I clinical trial
- LQT-1213 is currently being assessed in a P-I/II (Wave I) study. Part 1 of the study focuses on determining its effect on dofetilide-induced QTc prolongation in healthy adult subjects (n=28), while Part 2 evaluates the safety, tolerability, and pharmacokinetics (PK) in patients diagnosed with Long QT Syndrome types 1 (n=12), 2 (n=20), or 3 (n=12)
- LQT-1213 is a potent and selective SGK1 inhibitor, intended to treat Long QT Syndrome types 1, 2, and 3. It targets SGK1 to reduce prolonged QTc intervals and lower the risk of life-threatening arrhythmias.
IMM-1-104
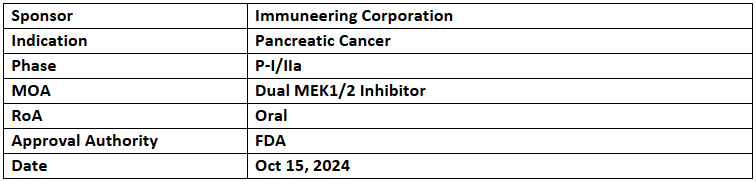
- The US FDA has granted Orphan Drug Designation (ODD) to IMM-1-104 for the treatment of pancreatic cancer.
- IMM-1-104 is being evaluated in a P-I/IIa study to assess its safety and anti-tumor activity in patients with RAS-mutated advanced or metastatic solid tumors.
- Initial data from the P-IIa part, involving IMM-1-104 in combination with modified gemcitabine/nab-paclitaxel in first-line pancreatic cancer patients, has been shared. The trial is also evaluating IMM-1-104 in combination with Folfirinox and as monotherapy, with additional data expected by the end of 2024.
ELA026

- The US FDA has granted ODD to ELA026 for the treatment of hemophagocytic lymphohistiocytosis (HLH)
- ELA026 is a first-in-class monoclonal antibody (mAb) that targets SIRP on myeloid cells and T lymphocytes to deplete pathological immune cells. It is under development for secondary hemophagocytic lymphohistiocytosis (sHLH)
MDL-101
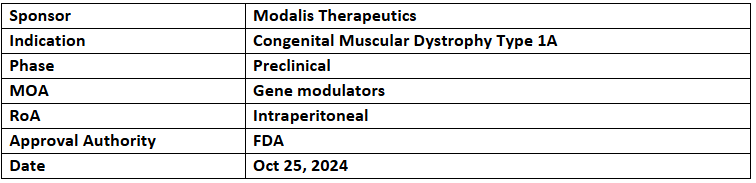
- The US FDA has granted ODD to the Modalis’ MDL-101 for the treatment of congenital muscular dystrophy type 1A (LAMA2-CMD)
- MDL-101 is an experimental therapy aimed at treating LAMA2-CMD through epigenetic editing. This therapy has the potential to provide a one-time, long-lasting treatment for individuals affected by LAMA2-CMD
- Modalis' CRISPR-GNDM technology offers an innovative approach to modulating gene expression without altering the patient's DNA. MDL-101 is designed to address an unmet medical need by stimulating the expression of the LAMA1 gene in muscle tissues, thereby compensating for the impaired function of LAMA2
CAP-002
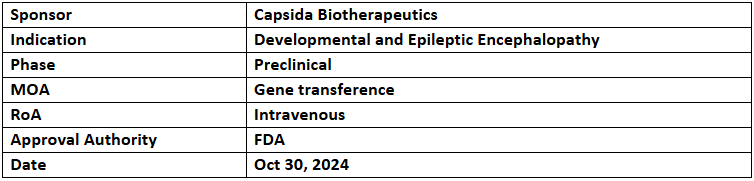
- The US FDA has granted ODD to CAP-002 for treating developmental and epileptic encephalopathy (DEE) caused by syntaxin-binding protein 1 (STXBP1) mutations
- CAP-002 is under IND-enabling evaluation, with IND filing anticipated in H1’25. The Preclinical pharmacology studies show that gene supplementation of STXBP1 in neurons can potentially treat and correct neurological phenotypes
- CAP-002 is a first-in-class gene therapy that uses Capsida's engineered capsids to achieve brain-wide STXBP1 expression while minimizing liver targeting
PPL-002
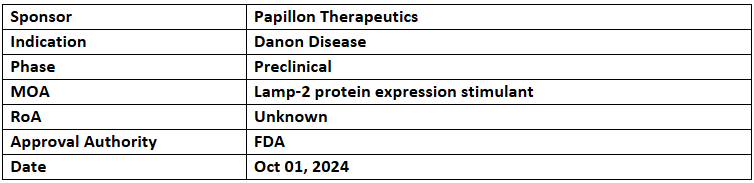
- The US FDA has granted ODD to PPL-002 for treating Danon disease
- Preclinical studies depicted that PPL-002 improved disease phenotype and targets multiple organ systems, offering potential to modify and reverse disease progression
- PPL-002 is a gene-modified CD34+ HSPC therapy developed to express functional Lamp-2 protein, deficient in patients with the condition. Its research is funded by the California Institute for Regenerative Medicine (CIRM)
NIDO-361
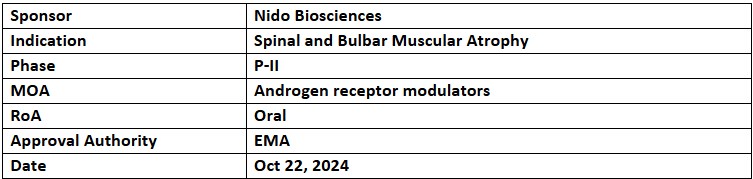
- The EMA has granted ODD to NIDO-361 for treating Spinal and Bulbar Muscular Atrophy (SBMA) or Kennedy’s disease
- NIDO-361 is being assessed under P-II trial, the 1EP of which includes changes in total and thigh lean muscle volume from baseline, while 2EPs are change from baseline in the SBMA functional rating scale, 6-minute walk test & grip strength assessment. Patients will take daily oral doses of NIDO-361 to determine optimal dose for P-III trials
- Nido has concluded enrollment of 54 patients in a P-II trial across the EU, UK & South Korea

Zilganersen
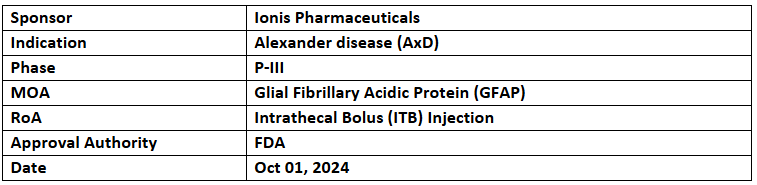
- The US FDA has granted FTD to Zilganersen (GFAP) for treating children & adults with Alexander disease (AxD). The primary results from the pivotal study of zilganersen are anticipated in H2’25
- Enrollment for the pivotal P-III clinical trials of Zilganersen in adults & children with AxD was completed earlier this year, involving 13 sites across eight countries
Liafensine
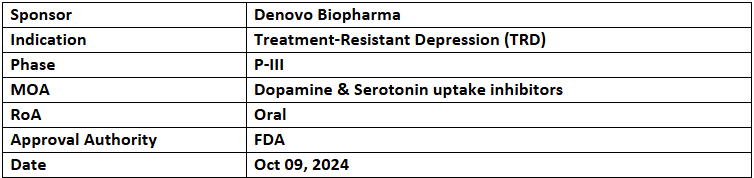
- The US FDA has granted FTD to liafensine (DB104) for treating treatment-resistant depression (TRD)
- Its P-IIb (ENLIGHTEN) study achieved all endpoints, including a significant MADRS score improvement at 6wks. Liafensine showed a favorable safety profile without common side effects (dissociation, respiratory depression, movement disorders, and metabolic dysfunction with morbid weight gain) as seen in other TRD drugs
Choline Chloride

- The US FDA has granted FTD to its investigational IV Choline Chloride therapy for adult and adolescent patients on parenteral support who cannot use oral or enteral nutrition
- Protara plans to evaluate IV Choline Chloride vs PBO in the registrational P-IIb/III (THRIVE-3) trial, with the 1EP as the change in plasma choline concentration from baseline. It will be commenced during Q1’25
VLS-1488
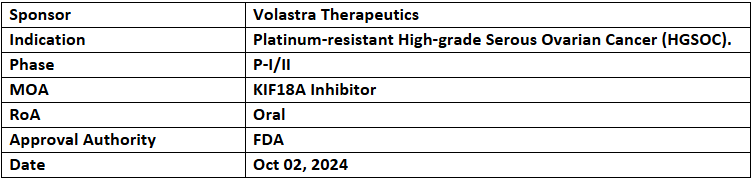
- The US FDA has granted FTD to VLS-1488 (KIF18A inhibitor) for treating Pt-resistant high-grade serous ovarian cancer (HGSOC).
- The P-I/II study of VLS-1488 is assessing its safety, tolerability & preliminary efficacy in patients with advanced tumors, HGSOC
Shigella4V (S4V)
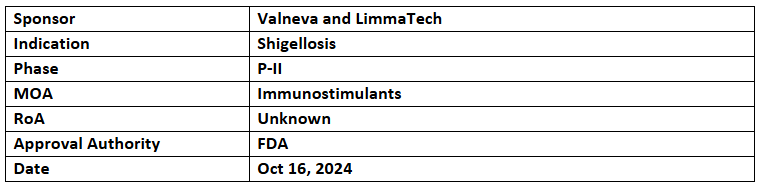
- The US FDA has granted FTD to Shigella4V (S4V), the advanced tetravalent shigellosis vaccine candidate, for which Valneva holds an exclusive worldwide license
- In Aug 2024, Valneva and LimmaTech formed a partnership to develop and commercialize S4V. Following positive P-I/II results, LimmaTech will handle P-II CHIM study in the US and P-II pediatric trial in LMICs, while Valneva will be responsible for further development & global commercialization
- The regulatory pathway for S4V will combine CHIM studies for initial adult approval and field efficacy studies to expand the indication to children
LP-184
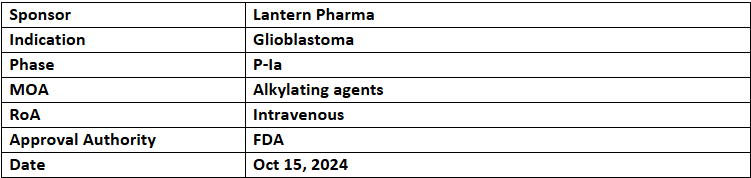
- The US FDA has granted FTD to LP-184, developed using RADR AI platform, for treating GBM. The company plans to use RADR for exploring LP-184 in combinations to control cancer progression
- LP-184 is in P-Ia assessment to find its safety, tolerability & MTD for various tumors, incl. GBM. Lantern will advance it as STAR-001 (via its subsidiary Starlight Therapeutics) for GBM plus other brain & CNS cancers after finding MTD
- The P-Ib/IIa study to evaluate the safety, PK & preliminary efficacy of LP-184 alone & in addition to spironolactone for rGBM is planned in late 2024 or early 2025. DNA damage markers, EGFR expression, MGMT status & DNA repair pathways will be analyzed as potential response predictors to plan future studies
HC-7366
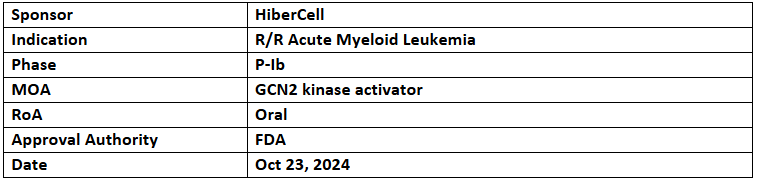
- The US FDA has granted FTD to HC-7366 (small molecule) for treating adults with r/r AML
- HC-7366 is currently being investigated under P-Ib trial for its safety, tolerability, preliminary efficacy & RP2D to treat r/r AML or MDS AML; the trial may expand to include monotx. & combinations based on these outcomes. It is also being assessed in another P-Ib/II trial combined with belzutifan for clear cell RCC
- HC-7366 works on the GCN2 kinase, responsible for integrated stress response (ISR) pathway which is utilized by the cancer cells for survival. It hyperactivates GCN2, leading to antitumor & immunomodulatory effects alone and combined with SoC in both solid & liquid tumor models
PP-007
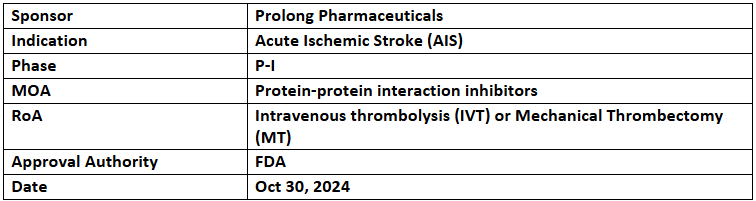
- The US FDA has granted FTD to PP-007 for treating acute ischemic stroke (AIS). PP-007 is currently undergoing evaluation for safety & efficacy in the ongoing HEMERA-1 study based in the US
- The P-I (HEMERA-1) study is a randomized, blinded, and contemporaneously controlled trial that assesses the safety, tolerability, efficacy, & PK of PP-007 in patients with AIS

Denifanstat
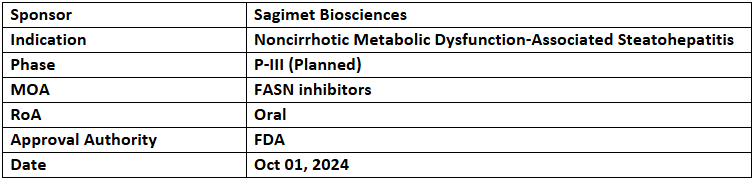
- The US FDA has granted BTD to denifanstat for treating noncirrhotic metabolic dysfunction-associated steatohepatitis (MASH) with mod. to advanced liver fibrosis, based on the data from P-IIb (FASCINATE-2) study
- Study showed improved 1EPs of MASH resolution without fibrosis worsening with ≥2-point reduction in NAFLD Activity Score (NAS) & a ≥2-point NAS reduction without fibrosis worsening. It also depicted improved fibrosis by ≥1 stage without MASH worsening and with more patients achieving ≥30% MRI-PDFF fat reduction
- The analysis of the ITT population showed significant results on primary & secondary liver biopsy endpoints. It was well tolerated, and its P-III study is planned in YE’24
Survodutide (BI 456906)
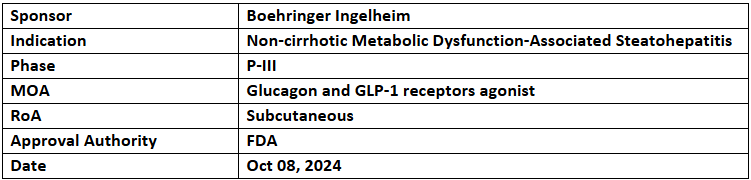
- BI has received the US FDA’s BTD for survodutide to treat non-cirrhotic MASH & mod. or advanced fibrosis (stages 2 or 3). BI has also begun 2 P-III (LIVERAGE & LIVERAGE-Cirrhosis) studies for the same
- The P-III (LIVERAGE; n=1,800 & LIVERAGE-Cirrhosis; n=1,590) studies will assess survodutide (6mg, weekly) vs PBO in adults with MASH & fibrosis (stages 2-3) as well as MASH cirrhosis (stage 4), respectively
- The 1EPs of LIVERAGE include MASH resolution without worsening fibrosis & 1pt. fibrosis improvement without worsening MASH post 52wks. (part 1) as well as first occurrence of liver-related events or mortality for 7yrs. (part 2). LIVERAGE-Cirrhosis will track first occurrence of mortality or liver-related events for 4.5yrs.
Felzartamab
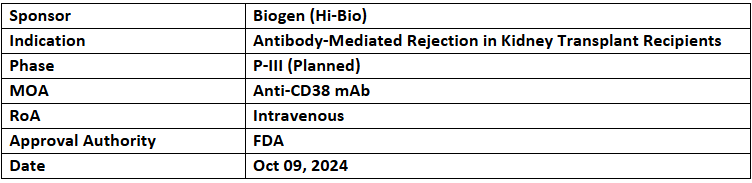
- The US FDA has granted BTD to felzartamab for treating late antibody-mediated rejection (AMR) without T-cell mediated rejection in kidney transplant individuals. The company anticipates a P-III study in AMR, IgAN & PMN during 2025
- BTD was based on the data from the study that validated clinical PoC. This data was published in the NEJM and presented as a late-breaking presentation at ERA 2024
- Felzartamab is a human mAb that targets CD38, a protein shown in mature plasma cells. Its P-II trials have been completed in AMR, PMN & IgAN. HI-Bio holds its exclusive development & commercial rights across all indications globally excl. China. Biogen acquired Human Immunology Biosciences (HI-Bio) in July 2024
Volixibat
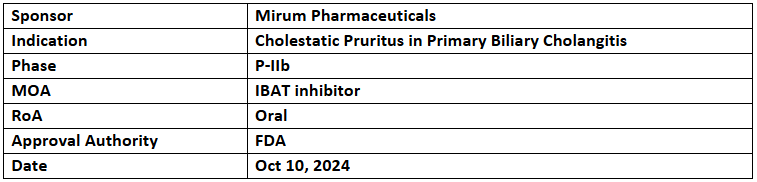
- The US FDA has awarded BTD to volixibat for the treatment of cholestatic pruritus in patients with primary biliary cholangitis (PBC)
- The designation was based on positive P-IIb (VANTAGE) trial, depicting significantly improved pruritus. The study's confirmatory phase is underway, with enrollment expected to complete in 2026
- Volixibat is being studied in P-IIb studies for primary sclerosing cholangitis (VISTAS) and primary biliary cholangitis (VANTAGE). Interim results from VANTAGE showed significantly improved pruritus, reduced serum bile acids & improved fatigue, with diarrhea as the most common mild to moderate AE
Sunvozertinib
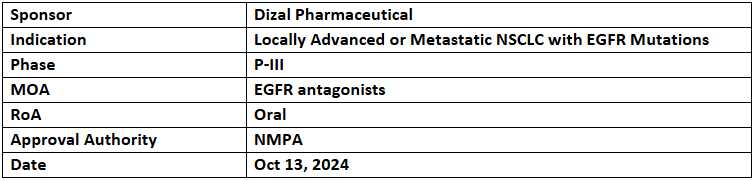
- The NMPA has designated sunvozertinib as a breakthrough therapy for the 1L treatment of locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations in treatment-naive patients
- The designation was based on combined analysis from P-I/II (WU-KONG1) & P-II (WU-KONG15) studies assessing sunvozertinib (oral) in Chinese patients, depicting a cORR of 78.6% & mPFS of 12.4mos. It is further being investigated under global P-III (WU-KONG28) trial in comparison with Pt doublet CTs for the same
- The company also highlighted data from part B of WU-KONG1 trial involving patients from Asia, EU, North & South America at ASCO 2024 (trial achieved its 1EP). Regulatory submissions are underway
LBL-024
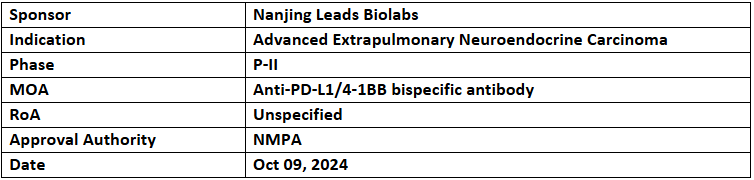
- The NMPA has granted BTD to LBL-024, an anti-PD-L1/4-1BB bispecific antibody, for treating advanced extrapulmonary neuroendocrine carcinoma (EP-NEC) in patients who have progressed after >2L of CT
- LBL-024 received BTD due to its strong clinical efficacy in advanced EP-NEC, showing more than double the ORR and OS as compared to existing treatments
HMI-115
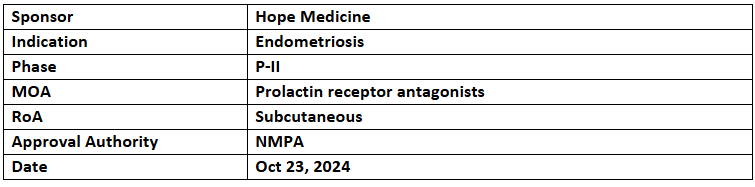
- HopeMed reported interim data from a global P-II trial assessing the safety & efficacy of HMI-115 (240mg, Q2W) vs PBO to treat women (n=142) with moderate to severe endometriosis-related pain for over 12wks. in the US, Poland & China
- In the first 102 patients analyzed, HMI-115 showed a 42% reduction in dysmenorrhea pain and a 50% mean reduction in non-menstrual pelvic pain; most of them had normal menstrual cycles without peri-menopausal symptoms or significant changes in bone density or hormone levels. It was well-tolerated without any serious TRAEs
- HMI-115 is a monoclonal antibody that works by blocking prolactin receptor for treating endometriosis. It has been designated as a breakthrough therapy by the China’s NMPA

Enhertu

- The sBLA for Enhertu has been accepted for priority review by the US FDA to treat HER2-low or ultralow metastatic breast cancer in patients who have received at least 1 endocrine therapy, with the decision expected during Q1’25
- The submission was based on P-III (DESTINY-Breast06) study assessing Enhertu (5.4mg/kg) vs investigator’s choice of CT (capecitabine, paclitaxel or nab-paclitaxel) to treat HR+/HER2-low or ultralow advanced or metastatic breast cancer
- Study demonstrated reduced risk of disease progression or death by 37% with mPFS of 13.2mos. vs 8.1mos. in the overall population. Similar results were seen in HER2-low & ultralow patients, with mPFS of 13.2mos. vs 8.1mos. & 13.2mos. vs 8.3mos., respectively
Calquence
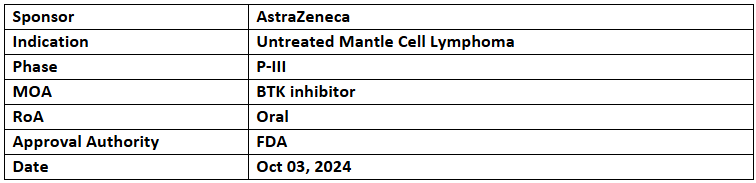
- The US FDA has accepted & granted priority review to sNDA of Calquence for treatment-naïve adults with mantle cell lymphoma. The decision is expected during Q1’25
- The submission was based on P-III (ECHO) study assessing the safety & efficacy of Calquence + bendamustine & rituximab (SoC) vs SoC to treat MCL adults (n=635, over 65yrs.). Data was featured at EHA 2024
- The study showed a 27% reduction in the disease progression or death risk with an mPFS of 66.4mos. vs 49.6mos. and an immature OS with favorable trends (assessment continues). In the pre-specified analysis, carried out at the time of pandemic censoring COVID-19-related deaths, showed improved PFS of 36% across both arms & a favorable OS trend towards the regimen
Sublocade
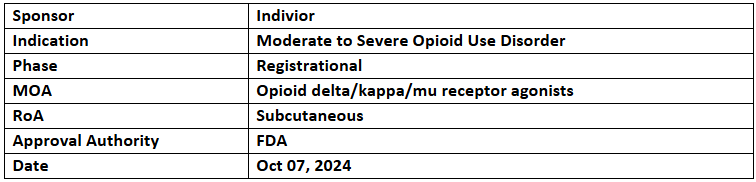
- The US FDA has granted Priority Review to its Prior Approval Supplement (PAS) for Sublocade, an extended-release buprenorphine injection for moderate to severe OUD, with a PDUFA date of Feb 7, 2025
- The PAS included efficacy & safety outcomes of Sublocade's new rapid induction protocol and PK profile after alternative injection sites
- The PAS proposes expanding Sublocade's label to include:
- additional injection sites (thigh, buttock, and upper arm)
- rapid induction protocol, reducing induction time from the current 7-day minimum on transmucosal buprenorphine (TM BUP) to a single TM BUP dose with 1hr. observation and allowing the second 300mg dose post 1wk. of initial 300mg injection
Pixclara (TLX101-CDx)
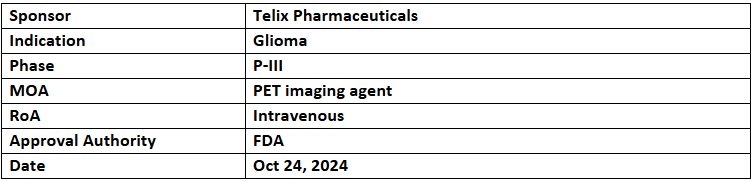
- The US FDA has accepted the NDA for TLX101-CDx (Pixclara), an imaging agent for glioma, with priority review and a PDUFA date of Apr 26, 2025. Pixclara also holds ODD & FTD
- TLX101-CDx (Pixclara) is a PET imaging agent targeting LAT and LAT2 membrane transport proteins. It will be explored as a companion diagnostic for TLX101, Telix's LAT1-targeting glioblastoma therapy under investigation in IPAX-2 and IPAX-Linz studies
Telitacicept
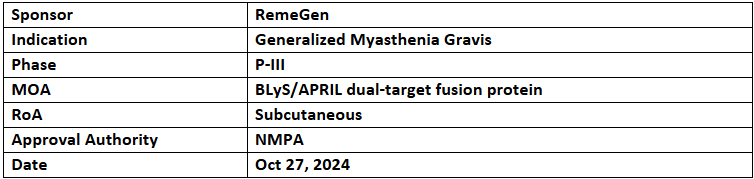
- RemeGen's BLA for telitacicept to treat generalized myasthenia gravis (gMG) has been accepted by China's NMPA with priority review. It also holds the NMPA’s BTD and the US FDA’s ODD as well as FTD
- Its P-III study in China showed effective reduction of clinical symptoms in gMG patients, demonstrating favorable efficacy and safety. RemeGen has enrolled the first patient across the US for its global P-III MG trial

Galinpepimut-S
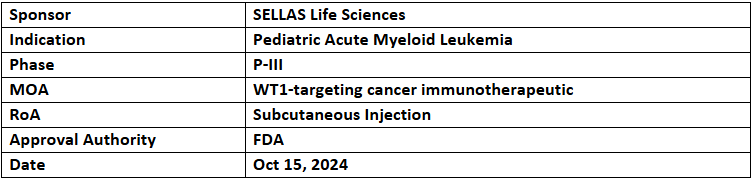
- The US FDA has granted RPDD to Galinpepimut-S (GPS) for treating pediatric acute myeloid leukemia (AML), making it eligible for priority review voucher if approved
- In adult AML patients in first complete remission, GPS showed a mOS of 67.6mos., a favorable safety, induced T-lymphocyte responses and improved outcomes in P-II trial. Results were positive in younger patients with mDFS & mOS not reached (over 50% were leukemia-free for >5yrs. Post treatment)
- GPS is currently being assessed under the P-III (REGAL) study for treating adults with AML, with interim analysis expected during Q4’24
BPM31510

- The US FDA has granted RPDD to BPM31510T for treating epidermolysis bullosa (EB), which makes it eligible for priority review voucher if approved
- BPM31510T was well-tolerated in its P-I study and showed its potential as an effective, easy-to-use topical therapy for EB. BPGbio is partnering with DEBRA of America to advance BPM31510T into P-II/III study, starting in 2025
- BPM31510T induces wound healing in EB patients by targeting cellular and mitochondrial processes responsible for inflammation, cell proliferation & tissue remodeling across multiple subtypes
PPL-001
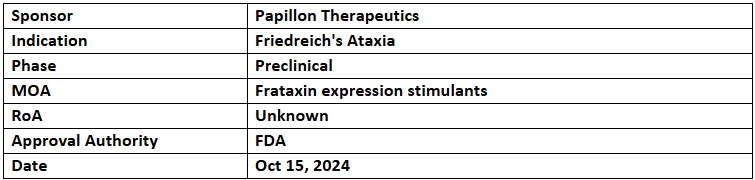
- The US FDA has granted RPDD to PPL-001 for treating Friedreich's ataxia, which makes it eligible for priority review voucher if approved. It was designated with the FDA’s ODD
- Preclinical studies depicted that PPL-001 improved disease phenotypes by targeting multiple organ systems, offering potential to reverse Friedreich’s ataxia progression. The research was funded by CIRM, FARA & NIH grants
- PPL-001 is a gene-corrected CD34+ HSPC therapy that uses targeted excision to correct the GAA repeat expansion in the FXN gene
PPL-002
.png)
- The US FDA has granted RPDD to PPL-002 for treating Danon disease, making it eligible for priority voucher if approved. It has also received the FDA’s ODD for the same
- Preclinical studies depicted that PPL-002 improved disease phenotype and targets multiple organ systems, offering potential to modify and reverse disease progression
- PPL-002 is a gene-modified CD34+ HSPC therapy developed to express functional Lamp-2 protein, deficient in patients with the condition. Its research is funded by the California Institute for Regenerative Medicine (CIRM)

Drug-device Combination Product
-
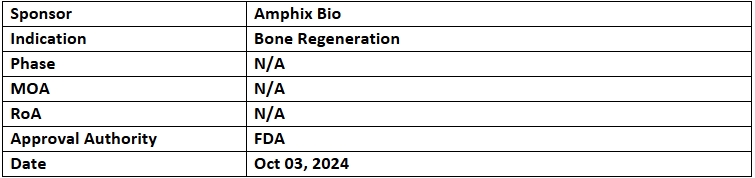
Amphix Bio has received the US FDA’s BDD for its drug-device combination product for bone regeneration, aimed at treating degenerative disc disease in TLIF procedures
NIO Lung Cancer Reveal

- Invenio Imaging's NIO Lung Cancer Reveal AI module has received the US FDA’s BDD to assist in evaluating bronchoscopic lung biopsies
- The NIO Lung Cancer Reveal identifies cancer-suspicious morphology in fresh biopsy images, but should not be used as the primary diagnosis; physicians must consider other clinical factors

HPC Cord Blood
.png)
- The US FDA has granted RMAT designation to Hematopoietic Progenitor Cell Cord Blood therapy (HPC Cord Blood) for treating long COVID-19 syndrome
- The designation was based on positive P-II study data that concluded full enrollment in 8mos. across the US, with the results pending. StemCyte is planning a P-III trial
- The company expects the US FDA’s approval by YE’24 for its BLA on HPC Cord Blood (RegeneCyte) to treat cancers, blood disorders, and immune deficiencies
ALLO-316
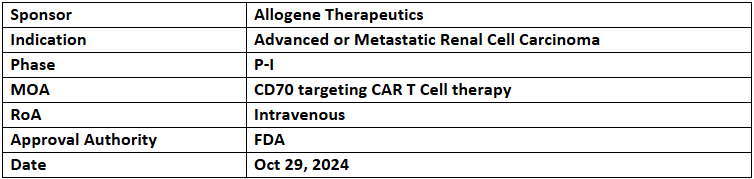
- The US FDA has granted RMAT designation to ALLO-316 (an AlloCAR T based on Dagger technology) for treating CD70+ advanced or metastatic renal cell carcinoma (RCC), based on the P-I (TRAVERSE) study
- The trial assesses the safety, tolerability & activity of ALLO-316 among RCC patients, with the initial readout highlighted at AACR 2023. Updated data will be presented at SITC 2024
- ALLO-316 has been previously designated with the US FDA’s FTD in Mar 2022 for the same
References
- PRNewswire
- Immuneering Corporation
- Electra Therapeutics
- Modalis Therapeutics
- Capsida Biotherapeutics
- Businesswire
- Ionis Pharmaceuticals
- Protara Therapeutics
- Volastra Therapeutics
- Valneva and LimmaTech
- Lantern Pharma
- GlobeNewsWire
- Sagimet Biosciences
- Biogen
- Mirum Pharmaceuticals
- Dizal Pharmaceutical
- Nanjing Leads Biolabs
- AstraZeneca and Daiichi Sankyo
- AstraZeneca
- RemeGen
- SELLAS Life Sciences
- BPGbio
- StemCyte
Related Post: New Drug Designations - September 2024
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














